After loading the IDA with Co2+ and the binding of the antibody, the Co2+ is oxidized with 0.03% H2O2 to Co3+, which will form an irreversible complex with the bound antibody.  G)OA[}g1l;v8m
Q.!QwnT[c{"-P+)J Zp!b^Qb1ZxDJx/g40EpZB^vvqNh)D#_4&. Even at high immobilization capacities, the XanTec bioanalytics high-affinity NiHC NTA sensor chip used showed no leaching effects, so Rmax values well below 10 RU could be evaluated (Fig.
G)OA[}g1l;v8m
Q.!QwnT[c{"-P+)J Zp!b^Qb1ZxDJx/g40EpZB^vvqNh)D#_4&. Even at high immobilization capacities, the XanTec bioanalytics high-affinity NiHC NTA sensor chip used showed no leaching effects, so Rmax values well below 10 RU could be evaluated (Fig.  Journal of Biological Chemistry, 295(25), 84808491. 0000013834 00000 n
Low flow rates (2-5 l/minute) can be used to achieve long contact times. For example, when analyzing antibody interactions, the antibody is a bivalent compound and has two binding sites which can both bind to a target molecule. 0000020126 00000 n
trailer
hbbd```b``WA$C)XDD%HT0X|+d&jT,n`RD Comparison between NiD (dextran based) and NiHC (linear polycarboxylate based sensor chip coating for reversible immobilization of His, Figure 2. 0000015244 00000 n
Binding and affinity data are key for characterizing molecular interactions, and with benchtop SPR, it has never been easier. %PDF-1.6
%
The great news is that Nicoya carries a wide-variety of sensor chips that are compatible with various tags! @;\qbQ|
W6TQYzg21Wet,,WYcu
endstream
endobj
84 0 obj
<>>>/Filter/Standard/Length 128/O(2#-&RR)/P -1340/R 4/StmF/StdCF/StrF/StdCF/U(%zC%/ )/V 4>>
endobj
85 0 obj
<>>>
endobj
86 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>>>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
87 0 obj
<>stream
To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. It is thought that the free Ni2+ will compete for the immobilized Ni2+ and so for the bound His-protein.
Journal of Biological Chemistry, 295(25), 84808491. 0000013834 00000 n
Low flow rates (2-5 l/minute) can be used to achieve long contact times. For example, when analyzing antibody interactions, the antibody is a bivalent compound and has two binding sites which can both bind to a target molecule. 0000020126 00000 n
trailer
hbbd```b``WA$C)XDD%HT0X|+d&jT,n`RD Comparison between NiD (dextran based) and NiHC (linear polycarboxylate based sensor chip coating for reversible immobilization of His, Figure 2. 0000015244 00000 n
Binding and affinity data are key for characterizing molecular interactions, and with benchtop SPR, it has never been easier. %PDF-1.6
%
The great news is that Nicoya carries a wide-variety of sensor chips that are compatible with various tags! @;\qbQ|
W6TQYzg21Wet,,WYcu
endstream
endobj
84 0 obj
<>>>/Filter/Standard/Length 128/O(2#-&RR)/P -1340/R 4/StmF/StdCF/StrF/StdCF/U(%zC%/ )/V 4>>
endobj
85 0 obj
<>>>
endobj
86 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>>>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
87 0 obj
<>stream
To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. It is thought that the free Ni2+ will compete for the immobilized Ni2+ and so for the bound His-protein.  This will result in a decrease in the association rate and a decrease in the dissociation rate compared to the intrinsic affinity of the interaction.
This will result in a decrease in the association rate and a decrease in the dissociation rate compared to the intrinsic affinity of the interaction. 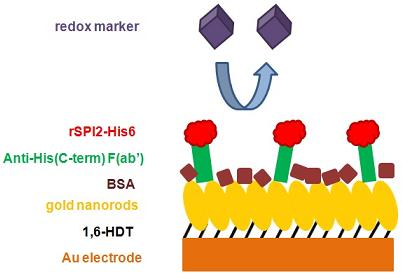 Because of the relatively low affinity between the His6-tag and the Ni2+-NTA-complex, continuous dissociation of the ligand from the sensor chip surface leads to unwanted baseline drifts. Published by Elsevier Inc. Side chains of cysteine, tyrosine, tryptophan and lysine on the surface of a protein may participate in binding to a chelated metal.
Because of the relatively low affinity between the His6-tag and the Ni2+-NTA-complex, continuous dissociation of the ligand from the sensor chip surface leads to unwanted baseline drifts. Published by Elsevier Inc. Side chains of cysteine, tyrosine, tryptophan and lysine on the surface of a protein may participate in binding to a chelated metal.  Some non-specific binding of crude ligand samples may be expected due to binding to the Ni2+(1). The most commonly used immobilization techniques (covalent immobilization, streptavidinbiotin) are irreversible in nature, which can afford excellent baseline stability but impose limitations throughput for slowly dissociating compounds or unstable targets. Mechanism of allosteric inhibition in the Plasmodium falciparum cGMP-dependent protein kinase. If the bivalent molecule is used as the analyte, it will bind to two ligand molecules. When looking at two different sized biomolecules, using the larger one as the analyte will ensure that the response signal is maximized, since SPR signals are dependent on the mass of analyte bound to the ligand. The binding of the histidines relies on a NTA-chelated nickel atom. At high concentration, there are fewer free binding sites thus higher dissociation constants. O~ 1L The use of EDTA in the eluent buffer helps to neutralize contamination metal ions that may be present in compounds used to prepare the buffer, but is dilute enough to not strip the nickel from the surface. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. Reversible immobilization (e.g., His-tagNi-NTA) is possible but typically precludes accurate quantification of slow dissociation kinetics due to baseline drift. 0000011837 00000 n
When working with carboxyl or NTA sensors, it is recommended to use the more negatively charged molecule as the analyte to reduce non-specific binding. Interaction/affinity map of His. The use of EDTA in the eluent buffer helps to neutralize contaminating metal ions that may be present in compounds used to prepare the buffer, but is diluted enough not to strip the nickel from the surface. (2005). High concentrations give multiphasic binding curves and a drop in binding levels during injection phase indicating a less stable binding. %%EOF
pH and ionic strength. Screening of low-molecular-weight fragments can identify hit compounds with better efficiency and physiological profiles than HTS2. 0000016365 00000 n
The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (1). Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. High-affinity adaptors for switchable recognition of histidine-tagged proteins. 0000020699 00000 n
Some non-specific binding of crude ligand samples may be expected due to binding to the Ni2+(1). The most commonly used immobilization techniques (covalent immobilization, streptavidinbiotin) are irreversible in nature, which can afford excellent baseline stability but impose limitations throughput for slowly dissociating compounds or unstable targets. Mechanism of allosteric inhibition in the Plasmodium falciparum cGMP-dependent protein kinase. If the bivalent molecule is used as the analyte, it will bind to two ligand molecules. When looking at two different sized biomolecules, using the larger one as the analyte will ensure that the response signal is maximized, since SPR signals are dependent on the mass of analyte bound to the ligand. The binding of the histidines relies on a NTA-chelated nickel atom. At high concentration, there are fewer free binding sites thus higher dissociation constants. O~ 1L The use of EDTA in the eluent buffer helps to neutralize contamination metal ions that may be present in compounds used to prepare the buffer, but is dilute enough to not strip the nickel from the surface. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. Reversible immobilization (e.g., His-tagNi-NTA) is possible but typically precludes accurate quantification of slow dissociation kinetics due to baseline drift. 0000011837 00000 n
When working with carboxyl or NTA sensors, it is recommended to use the more negatively charged molecule as the analyte to reduce non-specific binding. Interaction/affinity map of His. The use of EDTA in the eluent buffer helps to neutralize contaminating metal ions that may be present in compounds used to prepare the buffer, but is diluted enough not to strip the nickel from the surface. (2005). High concentrations give multiphasic binding curves and a drop in binding levels during injection phase indicating a less stable binding. %%EOF
pH and ionic strength. Screening of low-molecular-weight fragments can identify hit compounds with better efficiency and physiological profiles than HTS2. 0000016365 00000 n
The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (1). Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. High-affinity adaptors for switchable recognition of histidine-tagged proteins. 0000020699 00000 n
 Higher ligand concentrations may produce complex binding curves and less stable binding of the ligand when "non-his" sites with low affinity begin to participate in binding to the nickel atom. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Regenerable Biosensors for Small-Molecule Kinetic Characterization Using SPR. Although the affinity of these interactions are typically significantly lower than that commonly obtained with histidine tags. 0000008165 00000 n
Higher ligand concentrations may produce complex binding curves and less stable binding of the ligand when "non-his" sites with low affinity begin to participate in binding to the nickel atom. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Regenerable Biosensors for Small-Molecule Kinetic Characterization Using SPR. Although the affinity of these interactions are typically significantly lower than that commonly obtained with histidine tags. 0000008165 00000 n
 Retra, K., Irth, H., & van Muijlwijk-Koezen, J. E. (2010).
Retra, K., Irth, H., & van Muijlwijk-Koezen, J. E. (2010). 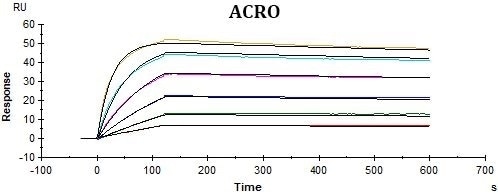 0000013860 00000 n
The difference between these two is that NTA forms a tetra coordinate complex with a metal ion leaving two free coordination sites while IDA forms a tri coordinate complex leaving three free coordination sites. 0000027734 00000 n
It is optimal to use the molecule with the least amount of non-specific binding as the ligand. In addition, at higher flow rates the dissociation rate is increased, which is a further support that rebinding is important in this system. Over 600 researchers worldwide are using OpenSPR to get the data reviewers are looking for. iBqE#H#G8c[:.3 eXoAI\g^uEqqv-,xN
~k8]FinC3,R'RgB3l7:6/77yie7IAiN'?CIzu.hj5.HbtUj(~msOh-pr)A>r?B:
0000002389 00000 n
0000013860 00000 n
The difference between these two is that NTA forms a tetra coordinate complex with a metal ion leaving two free coordination sites while IDA forms a tri coordinate complex leaving three free coordination sites. 0000027734 00000 n
It is optimal to use the molecule with the least amount of non-specific binding as the ligand. In addition, at higher flow rates the dissociation rate is increased, which is a further support that rebinding is important in this system. Over 600 researchers worldwide are using OpenSPR to get the data reviewers are looking for. iBqE#H#G8c[:.3 eXoAI\g^uEqqv-,xN
~k8]FinC3,R'RgB3l7:6/77yie7IAiN'?CIzu.hj5.HbtUj(~msOh-pr)A>r?B:
0000002389 00000 n
 The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as immobilization.
The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as immobilization.  First step is loading the sensor chip with NiCl2 (20 of 500 M with 20 l/min should give a baseline rise of 40 RU), followed by an injection with a His-tagged protein and subsequently by dissociation. The injection with EDTA (30 l at 10 l/min) strips all the His-tagged protein and Nickel, which is demonstrated by the lack of binding after injection with the same His-tagged protein. xref
0000004469 00000 n
First step is loading the sensor chip with NiCl2 (20 of 500 M with 20 l/min should give a baseline rise of 40 RU), followed by an injection with a His-tagged protein and subsequently by dissociation. The injection with EDTA (30 l at 10 l/min) strips all the His-tagged protein and Nickel, which is demonstrated by the lack of binding after injection with the same His-tagged protein. xref
0000004469 00000 n
 This allows repeated immobilization of sensitive ligands during extended FBDD campaigns, as NiHC chip surfaces are fully regenerable over many interaction cycles.
This allows repeated immobilization of sensitive ligands during extended FBDD campaigns, as NiHC chip surfaces are fully regenerable over many interaction cycles.  0000016956 00000 n
0000016956 00000 n
 State-of-the-art NTA sensor chips are based on carboxymethyl dextran (CMD) hydrogel modified with NTA groups. Gorshkova, I. I., Svitel, J., Razjouyan, F., & Schuck, P. (2008). Shepherd, C. A., Hopkins, A. L., & Navratilova, I. Through consistent research, XanTec has developed the widest portfolio of sensor chips available today, offering tailor-made solutions for almost any application. A direct binding assay was used to investigate the kinetics of cGMP (345 Da) and a cGMP analog (8-NBD-cGMP, 605 Da) binding to the cGMP-dependent protein kinase of Plasmodium falciparum (PfPKG), the malaria-associated parasite. So how do you choose which molecule should be the ligand and which should be the analyte? 79 41
The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as. Adding 8 M urea to the samples is possible without affecting the binding. v]_"m3\HVOJW.3p=1(X+/jf]"4B = G]~;/
hdN]ys -/zT9d @|{bKTqNu@iI6\6
(O9>4WBm18J"
i g!|vX${|l3,*GbM)lR
CA%$pm$7v:lB+1o./J0w2RzqYw,gz;p3G"^Z0Xd 2UoT%bb)F/(,8)B-~DNjIk=iDx-jc"| l 0000007233 00000 n
The Sensor chip NTA is more sensitive for changes in conditions than the NTA affinity columns (2),(3). XanTec bioanalytics specializes in manufacturing high quality sensor chips compatible with all major surface plasmon resonance (SPR) instrument brands on the market. 0000005690 00000 n
0000007786 00000 n
0000014167 00000 n
A key activity in small-molecule drug discovery is the characterization of compoundtarget interactions. 0000001138 00000 n
Some molecular interactions have more than one binding site. { Each has its own advantages and limitations, and while a universal immobilization procedure remains to be found, these strategies add to the immobilization toolbox that enables previously out-of-scope applications. So how do you choose which molecule should be the ligand and which should be the analyte? Langmuir, 24(20), 11577-11586. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. The affinity can also be affected by the buffer environment, e.g.
State-of-the-art NTA sensor chips are based on carboxymethyl dextran (CMD) hydrogel modified with NTA groups. Gorshkova, I. I., Svitel, J., Razjouyan, F., & Schuck, P. (2008). Shepherd, C. A., Hopkins, A. L., & Navratilova, I. Through consistent research, XanTec has developed the widest portfolio of sensor chips available today, offering tailor-made solutions for almost any application. A direct binding assay was used to investigate the kinetics of cGMP (345 Da) and a cGMP analog (8-NBD-cGMP, 605 Da) binding to the cGMP-dependent protein kinase of Plasmodium falciparum (PfPKG), the malaria-associated parasite. So how do you choose which molecule should be the ligand and which should be the analyte? 79 41
The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as. Adding 8 M urea to the samples is possible without affecting the binding. v]_"m3\HVOJW.3p=1(X+/jf]"4B = G]~;/
hdN]ys -/zT9d @|{bKTqNu@iI6\6
(O9>4WBm18J"
i g!|vX${|l3,*GbM)lR
CA%$pm$7v:lB+1o./J0w2RzqYw,gz;p3G"^Z0Xd 2UoT%bb)F/(,8)B-~DNjIk=iDx-jc"| l 0000007233 00000 n
The Sensor chip NTA is more sensitive for changes in conditions than the NTA affinity columns (2),(3). XanTec bioanalytics specializes in manufacturing high quality sensor chips compatible with all major surface plasmon resonance (SPR) instrument brands on the market. 0000005690 00000 n
0000007786 00000 n
0000014167 00000 n
A key activity in small-molecule drug discovery is the characterization of compoundtarget interactions. 0000001138 00000 n
Some molecular interactions have more than one binding site. { Each has its own advantages and limitations, and while a universal immobilization procedure remains to be found, these strategies add to the immobilization toolbox that enables previously out-of-scope applications. So how do you choose which molecule should be the ligand and which should be the analyte? Langmuir, 24(20), 11577-11586. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. The affinity can also be affected by the buffer environment, e.g.  0000001687 00000 n
0000001687 00000 n
 Surface plasmon resonance (SPR) is a flexible technique for this purpose, with a wide affinity range (micromoles to picomoles), low protein requirements, and the ability to characterize the kinetics of compound binding. The purity of the ligand isnt as important if the ligand has a tag and you are using a capture immobilization strategy. Our application specialists are available to answer any technical questions and will happily discuss your requirements. Navigating the Post-Covid Research Landscape Popup, is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. 0000013704 00000 n
Copyright 2021 Society for Laboratory Automation and Screening. Even if the amount of protein is limited, immobilization uses very small amounts (2-5 g) of protein per immobilization.
Surface plasmon resonance (SPR) is a flexible technique for this purpose, with a wide affinity range (micromoles to picomoles), low protein requirements, and the ability to characterize the kinetics of compound binding. The purity of the ligand isnt as important if the ligand has a tag and you are using a capture immobilization strategy. Our application specialists are available to answer any technical questions and will happily discuss your requirements. Navigating the Post-Covid Research Landscape Popup, is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. 0000013704 00000 n
Copyright 2021 Society for Laboratory Automation and Screening. Even if the amount of protein is limited, immobilization uses very small amounts (2-5 g) of protein per immobilization.
 0000010654 00000 n
%PDF-1.4
%
However, as with many other affinity tags (e.g. 0000001551 00000 n
By continuing you agree to the use of cookies. Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. The first binding site interaction will give a response and the second binding site interaction will stabilize the complex, but without changing the response. After washing with 0.5 M EDTA to remove unoxidized Cobalt and unbound antibody, the surface is ready to use. When carboxyl coupling is being used for immobilization, the purest protein should be used as the ligand to ensure that the surface only contains the target protein of interest. Progress in Biophysics and Molecular Biology, 116(2-3), 113-123. The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (3). A Simple Guide to Choosing the Best Ligand for Your SPR Experiment.
0000010654 00000 n
%PDF-1.4
%
However, as with many other affinity tags (e.g. 0000001551 00000 n
By continuing you agree to the use of cookies. Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. The first binding site interaction will give a response and the second binding site interaction will stabilize the complex, but without changing the response. After washing with 0.5 M EDTA to remove unoxidized Cobalt and unbound antibody, the surface is ready to use. When carboxyl coupling is being used for immobilization, the purest protein should be used as the ligand to ensure that the surface only contains the target protein of interest. Progress in Biophysics and Molecular Biology, 116(2-3), 113-123. The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (3). A Simple Guide to Choosing the Best Ligand for Your SPR Experiment.  10 mM HEPES, 150 mM NaCl, 50 M EDTA. 0000032461 00000 n
Immobilization via His-tags has also the advantage of orientating the ligand molecules in a homogeneous way and allowing the immobilization to be carried out without significant changing the pH or ionic strength during the coupling procedure. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. 0000001818 00000 n
PloS One, 13(7), e0200387. SPR biosensor technology is one of the primary biophysical methods to screen fragment libraries3 as newer instruments achieve sufficiently high signal-to-noise ratios to generate reliable data despite the low molecular weight and low affinity of many analytes. Afterwards the Cobalt is reduced with 1 M -mercaptoethanol to Co2+, which releases the antibody from the system. 79 0 obj <>
endobj
0000020206 00000 n
Interested in learning more about how Alto can accelerate your drug discovery? Journal of the American Chemical Society, 127(29), 10205-10215. Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. . Ligand concentration and contact time, typically in the range 1-15 minutes, are used to control the amount of bound ligand. Contact us today to make the most out of your assay and optimize your data. Despite the high affinity of this surface, the regeneration conditions (EDTA) are mild, and identical to those of the standard mono-NTA sensor chips. 0000009620 00000 n
As chelating agents, both NTA and IDA (iminodiacetic acid) are used (5) in immobilized metal-ion affinity chromatography (IMAC) (6).
10 mM HEPES, 150 mM NaCl, 50 M EDTA. 0000032461 00000 n
Immobilization via His-tags has also the advantage of orientating the ligand molecules in a homogeneous way and allowing the immobilization to be carried out without significant changing the pH or ionic strength during the coupling procedure. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. 0000001818 00000 n
PloS One, 13(7), e0200387. SPR biosensor technology is one of the primary biophysical methods to screen fragment libraries3 as newer instruments achieve sufficiently high signal-to-noise ratios to generate reliable data despite the low molecular weight and low affinity of many analytes. Afterwards the Cobalt is reduced with 1 M -mercaptoethanol to Co2+, which releases the antibody from the system. 79 0 obj <>
endobj
0000020206 00000 n
Interested in learning more about how Alto can accelerate your drug discovery? Journal of the American Chemical Society, 127(29), 10205-10215. Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. . Ligand concentration and contact time, typically in the range 1-15 minutes, are used to control the amount of bound ligand. Contact us today to make the most out of your assay and optimize your data. Despite the high affinity of this surface, the regeneration conditions (EDTA) are mild, and identical to those of the standard mono-NTA sensor chips. 0000009620 00000 n
As chelating agents, both NTA and IDA (iminodiacetic acid) are used (5) in immobilized metal-ion affinity chromatography (IMAC) (6).  For a case like this, the antibody should be used as the ligand, otherwise the measured data will not be accurate.
For a case like this, the antibody should be used as the ligand, otherwise the measured data will not be accurate.  If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. With XanTecs unique poly-NTA sensor chips (NiHC group) it is possible to establish higher immobilization levels compared to NTA-derivatized carboxymethyl dextran with the additional benefit of drastically reduced leaching, resulting in a practically drift-free baseline. Fragment screening by SPR and advanced application to GPCRs. A closer look at the His-protein association reveals that during the injection of the protein, there is a maximum in association followed by a slight dissociation during the injection. Discovery of fragments that target key interactions in the signal recognition particle (SRP) as potential leads for a new class of antibiotics. In previous approaches to establish FBDD assays using SPR, the ligand was covalently immobilized on the sensor surface with high immobilization levels to ensure that the protein bound stably to the sensor chip surface. If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. 0000008055 00000 n
startxref
Lata, S., Reichel, A., Brock, R., Tamp, R., & Piehler, J. 0000006785 00000 n
If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. With XanTecs unique poly-NTA sensor chips (NiHC group) it is possible to establish higher immobilization levels compared to NTA-derivatized carboxymethyl dextran with the additional benefit of drastically reduced leaching, resulting in a practically drift-free baseline. Fragment screening by SPR and advanced application to GPCRs. A closer look at the His-protein association reveals that during the injection of the protein, there is a maximum in association followed by a slight dissociation during the injection. Discovery of fragments that target key interactions in the signal recognition particle (SRP) as potential leads for a new class of antibiotics. In previous approaches to establish FBDD assays using SPR, the ligand was covalently immobilized on the sensor surface with high immobilization levels to ensure that the protein bound stably to the sensor chip surface. If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. 0000008055 00000 n
startxref
Lata, S., Reichel, A., Brock, R., Tamp, R., & Piehler, J. 0000006785 00000 n
 Surface Plasmon Resonance (SPR) is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. 0000002277 00000 n
Surface Plasmon Resonance (SPR) is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. 0000002277 00000 n
 0000012772 00000 n
0000012772 00000 n
 Faoro, C., Wilkinson-White, L., Kwan, A. H., & Ataide, S. F. (2018). 4). The effective use of polydentate high-affinity Ni-NTA surfaces in SPR measurements of small molecules is vividly demonstrated in a publication by Byun et al.6. When working with, . Both immobilization methods lack the possibility to remove the bound ligand from the sensor surface which is critical, for example, when working with GPCRs or other sensitive proteins which often denature over long screening campaigns. Byun, J. Fragment-based drug discovery (FBDD) has become a preferred alternative to high-throughput screening (HTS) to improve the discovery of small-molecule drug candidates. 0000035529 00000 n
0000039104 00000 n
0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 350 mM EDTA, 0.005% Surfactant P20, pH 8.3 or 50 mM Imidazole. H^5)"1!avA${d,bsAv3#
Ic} 0 '
endstream
endobj
startxref
0
%%EOF
132 0 obj
<>stream
biotin and antigen epitopes) the affinity may vary with the microenvironment created by moieties adjacent to the His-tag. Here we present our investigation of three immobilization strategies (dual-His-tagged target protein, His-tagged streptavidin, and switchavidin) that combine the robustness of irreversible immobilization with the flexibility of reversible immobilization. 0000012625 00000 n
Faoro, C., Wilkinson-White, L., Kwan, A. H., & Ataide, S. F. (2018). 4). The effective use of polydentate high-affinity Ni-NTA surfaces in SPR measurements of small molecules is vividly demonstrated in a publication by Byun et al.6. When working with, . Both immobilization methods lack the possibility to remove the bound ligand from the sensor surface which is critical, for example, when working with GPCRs or other sensitive proteins which often denature over long screening campaigns. Byun, J. Fragment-based drug discovery (FBDD) has become a preferred alternative to high-throughput screening (HTS) to improve the discovery of small-molecule drug candidates. 0000035529 00000 n
0000039104 00000 n
0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 350 mM EDTA, 0.005% Surfactant P20, pH 8.3 or 50 mM Imidazole. H^5)"1!avA${d,bsAv3#
Ic} 0 '
endstream
endobj
startxref
0
%%EOF
132 0 obj
<>stream
biotin and antigen epitopes) the affinity may vary with the microenvironment created by moieties adjacent to the His-tag. Here we present our investigation of three immobilization strategies (dual-His-tagged target protein, His-tagged streptavidin, and switchavidin) that combine the robustness of irreversible immobilization with the flexibility of reversible immobilization. 0000012625 00000 n
 Overlay plot of two sensorgrams comparing immobilization capacity and stability of His, Figure 3. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.
Overlay plot of two sensorgrams comparing immobilization capacity and stability of His, Figure 3. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.  0
2022 XanTec bioanalytics GmbH | | , A) Rigid carboxymethyled dextran hydrogel with single NTA groups with immobilized His, B) Flexible HC hydrogel forming poly-NTA chelating cages for high affinity immobilization of His, Figure 1. Thus, when only a part of the ligand sites are occupied, the binding seems to be more stable than at high concentrations. Adding 250 M EDTA to the ligand sample may reduce non-specific binding. This is explained by rebinding effects (1),(3). Suitable ligand concentrations are typically below 200 nM. 0000014279 00000 n
In some cases, it is possible to dissociate the bound His-protein with Ni2+. 0000020535 00000 n
[Mr{S)tQe%\A}Cr4,/:"xP0iaA{:!C|[S`iJ!z*zze{ ldKe>8{[&*! 0000000016 00000 n
Such applications are highlighted in two examples that greatly increased throughput for the kinetic characterization of potent kinase inhibitors and kinetic profiling of covalent inhibitors. Compared to the standard CMD-NTA chemistry, these new coatings, which are available in 30, 200, 1000 and 1500 nm thickness, can improve the stability of captured His6-tagged ligands by 2-3 magnitudes, matching the high affinity of the recently-developed Tris-NTA4. The great news is that Nicoya carries a wide-variety of, It is optimal to use the molecule with the least amount of non-specific binding as the ligand. Accelerate your drug discovery with Alto. 83 0 obj
<>
endobj
114 0 obj
<>/Encrypt 84 0 R/Filter/FlateDecode/ID[<1BDAE8F6437043D4AFEAA7EB88C81E25><9E98CB40D29342FC83FF4000C8985B37>]/Index[83 50]/Info 82 0 R/Length 130/Prev 516836/Root 85 0 R/Size 133/Type/XRef/W[1 3 1]>>stream
119 0 obj<>stream
Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. Low affinity binding needs continuous rebinding to generate stable binding. The affinity (KD 10-6 M (1) of this interaction is commonly sufficiently high to allow detailed analysis of subsequent analyte binding.
0
2022 XanTec bioanalytics GmbH | | , A) Rigid carboxymethyled dextran hydrogel with single NTA groups with immobilized His, B) Flexible HC hydrogel forming poly-NTA chelating cages for high affinity immobilization of His, Figure 1. Thus, when only a part of the ligand sites are occupied, the binding seems to be more stable than at high concentrations. Adding 250 M EDTA to the ligand sample may reduce non-specific binding. This is explained by rebinding effects (1),(3). Suitable ligand concentrations are typically below 200 nM. 0000014279 00000 n
In some cases, it is possible to dissociate the bound His-protein with Ni2+. 0000020535 00000 n
[Mr{S)tQe%\A}Cr4,/:"xP0iaA{:!C|[S`iJ!z*zze{ ldKe>8{[&*! 0000000016 00000 n
Such applications are highlighted in two examples that greatly increased throughput for the kinetic characterization of potent kinase inhibitors and kinetic profiling of covalent inhibitors. Compared to the standard CMD-NTA chemistry, these new coatings, which are available in 30, 200, 1000 and 1500 nm thickness, can improve the stability of captured His6-tagged ligands by 2-3 magnitudes, matching the high affinity of the recently-developed Tris-NTA4. The great news is that Nicoya carries a wide-variety of, It is optimal to use the molecule with the least amount of non-specific binding as the ligand. Accelerate your drug discovery with Alto. 83 0 obj
<>
endobj
114 0 obj
<>/Encrypt 84 0 R/Filter/FlateDecode/ID[<1BDAE8F6437043D4AFEAA7EB88C81E25><9E98CB40D29342FC83FF4000C8985B37>]/Index[83 50]/Info 82 0 R/Length 130/Prev 516836/Root 85 0 R/Size 133/Type/XRef/W[1 3 1]>>stream
119 0 obj<>stream
Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. Low affinity binding needs continuous rebinding to generate stable binding. The affinity (KD 10-6 M (1) of this interaction is commonly sufficiently high to allow detailed analysis of subsequent analyte binding.  Figures 2 and 3 are showing the much higher stability of a captured His6-tagged ligand (a protein A/G fusion protein) on XanTecs poly-NTA surface NiHC1000M than on NTA-derivatized CMD hydrogel (mono-NTA). Such drift effects can easily exceed the specific signal when screening small molecules and thus represent a major problem. Fill out the form below to download a product brochure. This field is for validation purposes and should be left unchanged. Surface Plasmon Resonance biosensor analysis as a useful tool in FBDD. A., Van, K., Huang, J., Henning, P., Franz, E., Akimoto, M., & Melacini, G. (2020). Hale (5) describes the oriented immobilization of an antibody via the C-terminal part of the heavy chain (in IMAC not sensor chips). endstream
endobj
80 0 obj<. 0000006368 00000 n
If a small compound is used as the analyte and a large protein is used as the ligand, relatively large amounts of ligand must be on the sensor chip before a significant signal upon binding of the small compound can be seen.
Figures 2 and 3 are showing the much higher stability of a captured His6-tagged ligand (a protein A/G fusion protein) on XanTecs poly-NTA surface NiHC1000M than on NTA-derivatized CMD hydrogel (mono-NTA). Such drift effects can easily exceed the specific signal when screening small molecules and thus represent a major problem. Fill out the form below to download a product brochure. This field is for validation purposes and should be left unchanged. Surface Plasmon Resonance biosensor analysis as a useful tool in FBDD. A., Van, K., Huang, J., Henning, P., Franz, E., Akimoto, M., & Melacini, G. (2020). Hale (5) describes the oriented immobilization of an antibody via the C-terminal part of the heavy chain (in IMAC not sensor chips). endstream
endobj
80 0 obj<. 0000006368 00000 n
If a small compound is used as the analyte and a large protein is used as the ligand, relatively large amounts of ligand must be on the sensor chip before a significant signal upon binding of the small compound can be seen. 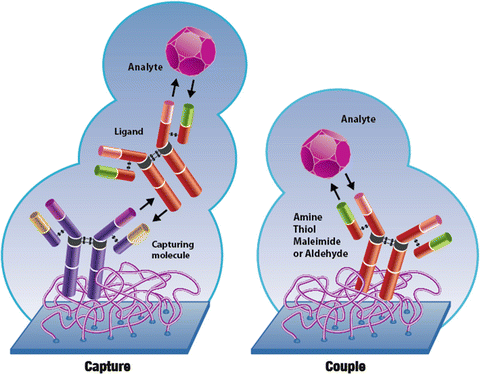 0000011552 00000 n
0000012184 00000 n
Drug Discovery Today: Technologies, 7(3), e181-e187. @@H
^g
5s/g@Y! wS*lNDQ#Ad23wA,
I[ <]>>
Sensor chip NTA (NTA: nitrilotriacetic acid) is designed to bind histidine-tagged molecules. Bayesian analysis of heterogeneity in the distribution of binding properties of immobilized surface sites. 0000020466 00000 n
To account for this well-known disadvantage, surface chemists at XanTec developed a poly-NTA sensor chip hydrogel coating based on a strongly hydrated and very flexible polycarboxylate polymer backbone. 0000003789 00000 n
However, a key requirement of SPR is the immobilization of the target protein to the surface of the sensor chip. (2014). For the best results, the histidine-tagged ligand should be purified prior to immobilization and prepared in eluent buffer. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.
0000011552 00000 n
0000012184 00000 n
Drug Discovery Today: Technologies, 7(3), e181-e187. @@H
^g
5s/g@Y! wS*lNDQ#Ad23wA,
I[ <]>>
Sensor chip NTA (NTA: nitrilotriacetic acid) is designed to bind histidine-tagged molecules. Bayesian analysis of heterogeneity in the distribution of binding properties of immobilized surface sites. 0000020466 00000 n
To account for this well-known disadvantage, surface chemists at XanTec developed a poly-NTA sensor chip hydrogel coating based on a strongly hydrated and very flexible polycarboxylate polymer backbone. 0000003789 00000 n
However, a key requirement of SPR is the immobilization of the target protein to the surface of the sensor chip. (2014). For the best results, the histidine-tagged ligand should be purified prior to immobilization and prepared in eluent buffer. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.  We use cookies to help provide and enhance our service and tailor content and ads. To make this group of sensitive molecules accessible to FBDD, various attempts have been made to immobilize them reversibly via affinity based His6/nickel-nitrilotriacetic acid (NTA) coupling (Fig. 1). If you are having trouble with nonspecific binding, here are. Alternatively, biotinylated proteins were immobilized on streptavidin coated sensor surfaces with the inherent drawback that the analyte could non-specifically interact with the streptavidin. O'Shannessey (2) tested three buffers (Tris, Hepes, phosphate) and concluded that Hepes gives the best reproducible and sensitive results.
We use cookies to help provide and enhance our service and tailor content and ads. To make this group of sensitive molecules accessible to FBDD, various attempts have been made to immobilize them reversibly via affinity based His6/nickel-nitrilotriacetic acid (NTA) coupling (Fig. 1). If you are having trouble with nonspecific binding, here are. Alternatively, biotinylated proteins were immobilized on streptavidin coated sensor surfaces with the inherent drawback that the analyte could non-specifically interact with the streptavidin. O'Shannessey (2) tested three buffers (Tris, Hepes, phosphate) and concluded that Hepes gives the best reproducible and sensitive results.  0000005086 00000 n
0000005086 00000 n
 Copyright 2022 Elsevier B.V. or its licensors or contributors. Read our brochure to learn more! If you are having trouble with nonspecific binding, here are 4 ways to reduce non-specific binding.
Copyright 2022 Elsevier B.V. or its licensors or contributors. Read our brochure to learn more! If you are having trouble with nonspecific binding, here are 4 ways to reduce non-specific binding.
 G)OA[}g1l;v8m
Q.!QwnT[c{"-P+)J Zp!b^Qb1ZxDJx/g40EpZB^vvqNh)D#_4&. Even at high immobilization capacities, the XanTec bioanalytics high-affinity NiHC NTA sensor chip used showed no leaching effects, so Rmax values well below 10 RU could be evaluated (Fig.
G)OA[}g1l;v8m
Q.!QwnT[c{"-P+)J Zp!b^Qb1ZxDJx/g40EpZB^vvqNh)D#_4&. Even at high immobilization capacities, the XanTec bioanalytics high-affinity NiHC NTA sensor chip used showed no leaching effects, so Rmax values well below 10 RU could be evaluated (Fig.  Journal of Biological Chemistry, 295(25), 84808491. 0000013834 00000 n
Low flow rates (2-5 l/minute) can be used to achieve long contact times. For example, when analyzing antibody interactions, the antibody is a bivalent compound and has two binding sites which can both bind to a target molecule. 0000020126 00000 n
trailer
hbbd```b``WA$C)XDD%HT0X|+d&jT,n`RD Comparison between NiD (dextran based) and NiHC (linear polycarboxylate based sensor chip coating for reversible immobilization of His, Figure 2. 0000015244 00000 n
Binding and affinity data are key for characterizing molecular interactions, and with benchtop SPR, it has never been easier. %PDF-1.6
%
The great news is that Nicoya carries a wide-variety of sensor chips that are compatible with various tags! @;\qbQ|
W6TQYzg21Wet,,WYcu
endstream
endobj
84 0 obj
<>>>/Filter/Standard/Length 128/O(2#-&RR)/P -1340/R 4/StmF/StdCF/StrF/StdCF/U(%zC%/ )/V 4>>
endobj
85 0 obj
<>>>
endobj
86 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>>>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
87 0 obj
<>stream
To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. It is thought that the free Ni2+ will compete for the immobilized Ni2+ and so for the bound His-protein.
Journal of Biological Chemistry, 295(25), 84808491. 0000013834 00000 n
Low flow rates (2-5 l/minute) can be used to achieve long contact times. For example, when analyzing antibody interactions, the antibody is a bivalent compound and has two binding sites which can both bind to a target molecule. 0000020126 00000 n
trailer
hbbd```b``WA$C)XDD%HT0X|+d&jT,n`RD Comparison between NiD (dextran based) and NiHC (linear polycarboxylate based sensor chip coating for reversible immobilization of His, Figure 2. 0000015244 00000 n
Binding and affinity data are key for characterizing molecular interactions, and with benchtop SPR, it has never been easier. %PDF-1.6
%
The great news is that Nicoya carries a wide-variety of sensor chips that are compatible with various tags! @;\qbQ|
W6TQYzg21Wet,,WYcu
endstream
endobj
84 0 obj
<>>>/Filter/Standard/Length 128/O(2#-&RR)/P -1340/R 4/StmF/StdCF/StrF/StdCF/U(%zC%/ )/V 4>>
endobj
85 0 obj
<>>>
endobj
86 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>>>/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
87 0 obj
<>stream
To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. It is thought that the free Ni2+ will compete for the immobilized Ni2+ and so for the bound His-protein.  This will result in a decrease in the association rate and a decrease in the dissociation rate compared to the intrinsic affinity of the interaction.
This will result in a decrease in the association rate and a decrease in the dissociation rate compared to the intrinsic affinity of the interaction.  Because of the relatively low affinity between the His6-tag and the Ni2+-NTA-complex, continuous dissociation of the ligand from the sensor chip surface leads to unwanted baseline drifts. Published by Elsevier Inc. Side chains of cysteine, tyrosine, tryptophan and lysine on the surface of a protein may participate in binding to a chelated metal.
Because of the relatively low affinity between the His6-tag and the Ni2+-NTA-complex, continuous dissociation of the ligand from the sensor chip surface leads to unwanted baseline drifts. Published by Elsevier Inc. Side chains of cysteine, tyrosine, tryptophan and lysine on the surface of a protein may participate in binding to a chelated metal.  Some non-specific binding of crude ligand samples may be expected due to binding to the Ni2+(1). The most commonly used immobilization techniques (covalent immobilization, streptavidinbiotin) are irreversible in nature, which can afford excellent baseline stability but impose limitations throughput for slowly dissociating compounds or unstable targets. Mechanism of allosteric inhibition in the Plasmodium falciparum cGMP-dependent protein kinase. If the bivalent molecule is used as the analyte, it will bind to two ligand molecules. When looking at two different sized biomolecules, using the larger one as the analyte will ensure that the response signal is maximized, since SPR signals are dependent on the mass of analyte bound to the ligand. The binding of the histidines relies on a NTA-chelated nickel atom. At high concentration, there are fewer free binding sites thus higher dissociation constants. O~ 1L The use of EDTA in the eluent buffer helps to neutralize contamination metal ions that may be present in compounds used to prepare the buffer, but is dilute enough to not strip the nickel from the surface. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. Reversible immobilization (e.g., His-tagNi-NTA) is possible but typically precludes accurate quantification of slow dissociation kinetics due to baseline drift. 0000011837 00000 n
When working with carboxyl or NTA sensors, it is recommended to use the more negatively charged molecule as the analyte to reduce non-specific binding. Interaction/affinity map of His. The use of EDTA in the eluent buffer helps to neutralize contaminating metal ions that may be present in compounds used to prepare the buffer, but is diluted enough not to strip the nickel from the surface. (2005). High concentrations give multiphasic binding curves and a drop in binding levels during injection phase indicating a less stable binding. %%EOF
pH and ionic strength. Screening of low-molecular-weight fragments can identify hit compounds with better efficiency and physiological profiles than HTS2. 0000016365 00000 n
The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (1). Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. High-affinity adaptors for switchable recognition of histidine-tagged proteins. 0000020699 00000 n
Some non-specific binding of crude ligand samples may be expected due to binding to the Ni2+(1). The most commonly used immobilization techniques (covalent immobilization, streptavidinbiotin) are irreversible in nature, which can afford excellent baseline stability but impose limitations throughput for slowly dissociating compounds or unstable targets. Mechanism of allosteric inhibition in the Plasmodium falciparum cGMP-dependent protein kinase. If the bivalent molecule is used as the analyte, it will bind to two ligand molecules. When looking at two different sized biomolecules, using the larger one as the analyte will ensure that the response signal is maximized, since SPR signals are dependent on the mass of analyte bound to the ligand. The binding of the histidines relies on a NTA-chelated nickel atom. At high concentration, there are fewer free binding sites thus higher dissociation constants. O~ 1L The use of EDTA in the eluent buffer helps to neutralize contamination metal ions that may be present in compounds used to prepare the buffer, but is dilute enough to not strip the nickel from the surface. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. Reversible immobilization (e.g., His-tagNi-NTA) is possible but typically precludes accurate quantification of slow dissociation kinetics due to baseline drift. 0000011837 00000 n
When working with carboxyl or NTA sensors, it is recommended to use the more negatively charged molecule as the analyte to reduce non-specific binding. Interaction/affinity map of His. The use of EDTA in the eluent buffer helps to neutralize contaminating metal ions that may be present in compounds used to prepare the buffer, but is diluted enough not to strip the nickel from the surface. (2005). High concentrations give multiphasic binding curves and a drop in binding levels during injection phase indicating a less stable binding. %%EOF
pH and ionic strength. Screening of low-molecular-weight fragments can identify hit compounds with better efficiency and physiological profiles than HTS2. 0000016365 00000 n
The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (1). Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. High-affinity adaptors for switchable recognition of histidine-tagged proteins. 0000020699 00000 n
 Higher ligand concentrations may produce complex binding curves and less stable binding of the ligand when "non-his" sites with low affinity begin to participate in binding to the nickel atom. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Regenerable Biosensors for Small-Molecule Kinetic Characterization Using SPR. Although the affinity of these interactions are typically significantly lower than that commonly obtained with histidine tags. 0000008165 00000 n
Higher ligand concentrations may produce complex binding curves and less stable binding of the ligand when "non-his" sites with low affinity begin to participate in binding to the nickel atom. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Regenerable Biosensors for Small-Molecule Kinetic Characterization Using SPR. Although the affinity of these interactions are typically significantly lower than that commonly obtained with histidine tags. 0000008165 00000 n
 Retra, K., Irth, H., & van Muijlwijk-Koezen, J. E. (2010).
Retra, K., Irth, H., & van Muijlwijk-Koezen, J. E. (2010).  0000013860 00000 n
The difference between these two is that NTA forms a tetra coordinate complex with a metal ion leaving two free coordination sites while IDA forms a tri coordinate complex leaving three free coordination sites. 0000027734 00000 n
It is optimal to use the molecule with the least amount of non-specific binding as the ligand. In addition, at higher flow rates the dissociation rate is increased, which is a further support that rebinding is important in this system. Over 600 researchers worldwide are using OpenSPR to get the data reviewers are looking for. iBqE#H#G8c[:.3 eXoAI\g^uEqqv-,xN
~k8]FinC3,R'RgB3l7:6/77yie7IAiN'?CIzu.hj5.HbtUj(~msOh-pr)A>r?B:
0000002389 00000 n
0000013860 00000 n
The difference between these two is that NTA forms a tetra coordinate complex with a metal ion leaving two free coordination sites while IDA forms a tri coordinate complex leaving three free coordination sites. 0000027734 00000 n
It is optimal to use the molecule with the least amount of non-specific binding as the ligand. In addition, at higher flow rates the dissociation rate is increased, which is a further support that rebinding is important in this system. Over 600 researchers worldwide are using OpenSPR to get the data reviewers are looking for. iBqE#H#G8c[:.3 eXoAI\g^uEqqv-,xN
~k8]FinC3,R'RgB3l7:6/77yie7IAiN'?CIzu.hj5.HbtUj(~msOh-pr)A>r?B:
0000002389 00000 n
 The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as immobilization.
The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as immobilization.  First step is loading the sensor chip with NiCl2 (20 of 500 M with 20 l/min should give a baseline rise of 40 RU), followed by an injection with a His-tagged protein and subsequently by dissociation. The injection with EDTA (30 l at 10 l/min) strips all the His-tagged protein and Nickel, which is demonstrated by the lack of binding after injection with the same His-tagged protein. xref
0000004469 00000 n
First step is loading the sensor chip with NiCl2 (20 of 500 M with 20 l/min should give a baseline rise of 40 RU), followed by an injection with a His-tagged protein and subsequently by dissociation. The injection with EDTA (30 l at 10 l/min) strips all the His-tagged protein and Nickel, which is demonstrated by the lack of binding after injection with the same His-tagged protein. xref
0000004469 00000 n
 This allows repeated immobilization of sensitive ligands during extended FBDD campaigns, as NiHC chip surfaces are fully regenerable over many interaction cycles.
This allows repeated immobilization of sensitive ligands during extended FBDD campaigns, as NiHC chip surfaces are fully regenerable over many interaction cycles.  0000016956 00000 n
0000016956 00000 n
 State-of-the-art NTA sensor chips are based on carboxymethyl dextran (CMD) hydrogel modified with NTA groups. Gorshkova, I. I., Svitel, J., Razjouyan, F., & Schuck, P. (2008). Shepherd, C. A., Hopkins, A. L., & Navratilova, I. Through consistent research, XanTec has developed the widest portfolio of sensor chips available today, offering tailor-made solutions for almost any application. A direct binding assay was used to investigate the kinetics of cGMP (345 Da) and a cGMP analog (8-NBD-cGMP, 605 Da) binding to the cGMP-dependent protein kinase of Plasmodium falciparum (PfPKG), the malaria-associated parasite. So how do you choose which molecule should be the ligand and which should be the analyte? 79 41
The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as. Adding 8 M urea to the samples is possible without affecting the binding. v]_"m3\HVOJW.3p=1(X+/jf]"4B = G]~;/
hdN]ys -/zT9d @|{bKTqNu@iI6\6
(O9>4WBm18J"
i g!|vX${|l3,*GbM)lR
CA%$pm$7v:lB+1o./J0w2RzqYw,gz;p3G"^Z0Xd 2UoT%bb)F/(,8)B-~DNjIk=iDx-jc"| l 0000007233 00000 n
The Sensor chip NTA is more sensitive for changes in conditions than the NTA affinity columns (2),(3). XanTec bioanalytics specializes in manufacturing high quality sensor chips compatible with all major surface plasmon resonance (SPR) instrument brands on the market. 0000005690 00000 n
0000007786 00000 n
0000014167 00000 n
A key activity in small-molecule drug discovery is the characterization of compoundtarget interactions. 0000001138 00000 n
Some molecular interactions have more than one binding site. { Each has its own advantages and limitations, and while a universal immobilization procedure remains to be found, these strategies add to the immobilization toolbox that enables previously out-of-scope applications. So how do you choose which molecule should be the ligand and which should be the analyte? Langmuir, 24(20), 11577-11586. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. The affinity can also be affected by the buffer environment, e.g.
State-of-the-art NTA sensor chips are based on carboxymethyl dextran (CMD) hydrogel modified with NTA groups. Gorshkova, I. I., Svitel, J., Razjouyan, F., & Schuck, P. (2008). Shepherd, C. A., Hopkins, A. L., & Navratilova, I. Through consistent research, XanTec has developed the widest portfolio of sensor chips available today, offering tailor-made solutions for almost any application. A direct binding assay was used to investigate the kinetics of cGMP (345 Da) and a cGMP analog (8-NBD-cGMP, 605 Da) binding to the cGMP-dependent protein kinase of Plasmodium falciparum (PfPKG), the malaria-associated parasite. So how do you choose which molecule should be the ligand and which should be the analyte? 79 41
The attachment of one of the interactants to the sensor chip surface in the form of a covalent bond or transient by means of capturing is known as. Adding 8 M urea to the samples is possible without affecting the binding. v]_"m3\HVOJW.3p=1(X+/jf]"4B = G]~;/
hdN]ys -/zT9d @|{bKTqNu@iI6\6
(O9>4WBm18J"
i g!|vX${|l3,*GbM)lR
CA%$pm$7v:lB+1o./J0w2RzqYw,gz;p3G"^Z0Xd 2UoT%bb)F/(,8)B-~DNjIk=iDx-jc"| l 0000007233 00000 n
The Sensor chip NTA is more sensitive for changes in conditions than the NTA affinity columns (2),(3). XanTec bioanalytics specializes in manufacturing high quality sensor chips compatible with all major surface plasmon resonance (SPR) instrument brands on the market. 0000005690 00000 n
0000007786 00000 n
0000014167 00000 n
A key activity in small-molecule drug discovery is the characterization of compoundtarget interactions. 0000001138 00000 n
Some molecular interactions have more than one binding site. { Each has its own advantages and limitations, and while a universal immobilization procedure remains to be found, these strategies add to the immobilization toolbox that enables previously out-of-scope applications. So how do you choose which molecule should be the ligand and which should be the analyte? Langmuir, 24(20), 11577-11586. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. The affinity can also be affected by the buffer environment, e.g.  0000001687 00000 n
0000001687 00000 n
 0000010654 00000 n
%PDF-1.4
%
However, as with many other affinity tags (e.g. 0000001551 00000 n
By continuing you agree to the use of cookies. Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. The first binding site interaction will give a response and the second binding site interaction will stabilize the complex, but without changing the response. After washing with 0.5 M EDTA to remove unoxidized Cobalt and unbound antibody, the surface is ready to use. When carboxyl coupling is being used for immobilization, the purest protein should be used as the ligand to ensure that the surface only contains the target protein of interest. Progress in Biophysics and Molecular Biology, 116(2-3), 113-123. The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (3). A Simple Guide to Choosing the Best Ligand for Your SPR Experiment.
0000010654 00000 n
%PDF-1.4
%
However, as with many other affinity tags (e.g. 0000001551 00000 n
By continuing you agree to the use of cookies. Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. To set up your SPR experiment for the best results, follow this easy guide on how to choose which binding partner should be the ligand and which should be the analyte. The first binding site interaction will give a response and the second binding site interaction will stabilize the complex, but without changing the response. After washing with 0.5 M EDTA to remove unoxidized Cobalt and unbound antibody, the surface is ready to use. When carboxyl coupling is being used for immobilization, the purest protein should be used as the ligand to ensure that the surface only contains the target protein of interest. Progress in Biophysics and Molecular Biology, 116(2-3), 113-123. The use of two buffers is to avoid binding to free metal ions to the his-tagged proteins (3). A Simple Guide to Choosing the Best Ligand for Your SPR Experiment.  10 mM HEPES, 150 mM NaCl, 50 M EDTA. 0000032461 00000 n
Immobilization via His-tags has also the advantage of orientating the ligand molecules in a homogeneous way and allowing the immobilization to be carried out without significant changing the pH or ionic strength during the coupling procedure. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. 0000001818 00000 n
PloS One, 13(7), e0200387. SPR biosensor technology is one of the primary biophysical methods to screen fragment libraries3 as newer instruments achieve sufficiently high signal-to-noise ratios to generate reliable data despite the low molecular weight and low affinity of many analytes. Afterwards the Cobalt is reduced with 1 M -mercaptoethanol to Co2+, which releases the antibody from the system. 79 0 obj <>
endobj
0000020206 00000 n
Interested in learning more about how Alto can accelerate your drug discovery? Journal of the American Chemical Society, 127(29), 10205-10215. Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. . Ligand concentration and contact time, typically in the range 1-15 minutes, are used to control the amount of bound ligand. Contact us today to make the most out of your assay and optimize your data. Despite the high affinity of this surface, the regeneration conditions (EDTA) are mild, and identical to those of the standard mono-NTA sensor chips. 0000009620 00000 n
As chelating agents, both NTA and IDA (iminodiacetic acid) are used (5) in immobilized metal-ion affinity chromatography (IMAC) (6).
10 mM HEPES, 150 mM NaCl, 50 M EDTA. 0000032461 00000 n
Immobilization via His-tags has also the advantage of orientating the ligand molecules in a homogeneous way and allowing the immobilization to be carried out without significant changing the pH or ionic strength during the coupling procedure. The presence of tags makes it easier to immobilize proteins to the surface, and often results in higher activity of the ligand because it is oriented in way that ensures the binding site is accessible. If you are working with a protein that already contains a tag (for example biotin, his-tag, GST-tag, etc) it is helpful to use the tagged protein as the ligand. 0000001818 00000 n
PloS One, 13(7), e0200387. SPR biosensor technology is one of the primary biophysical methods to screen fragment libraries3 as newer instruments achieve sufficiently high signal-to-noise ratios to generate reliable data despite the low molecular weight and low affinity of many analytes. Afterwards the Cobalt is reduced with 1 M -mercaptoethanol to Co2+, which releases the antibody from the system. 79 0 obj <>
endobj
0000020206 00000 n
Interested in learning more about how Alto can accelerate your drug discovery? Journal of the American Chemical Society, 127(29), 10205-10215. Choosing the best ligand for your experiment will help simplify immobilization, maximize signal to noise and minimize non-specific binding. . Ligand concentration and contact time, typically in the range 1-15 minutes, are used to control the amount of bound ligand. Contact us today to make the most out of your assay and optimize your data. Despite the high affinity of this surface, the regeneration conditions (EDTA) are mild, and identical to those of the standard mono-NTA sensor chips. 0000009620 00000 n
As chelating agents, both NTA and IDA (iminodiacetic acid) are used (5) in immobilized metal-ion affinity chromatography (IMAC) (6).  If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. With XanTecs unique poly-NTA sensor chips (NiHC group) it is possible to establish higher immobilization levels compared to NTA-derivatized carboxymethyl dextran with the additional benefit of drastically reduced leaching, resulting in a practically drift-free baseline. Fragment screening by SPR and advanced application to GPCRs. A closer look at the His-protein association reveals that during the injection of the protein, there is a maximum in association followed by a slight dissociation during the injection. Discovery of fragments that target key interactions in the signal recognition particle (SRP) as potential leads for a new class of antibiotics. In previous approaches to establish FBDD assays using SPR, the ligand was covalently immobilized on the sensor surface with high immobilization levels to ensure that the protein bound stably to the sensor chip surface. If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. 0000008055 00000 n
startxref
Lata, S., Reichel, A., Brock, R., Tamp, R., & Piehler, J. 0000006785 00000 n
If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. With XanTecs unique poly-NTA sensor chips (NiHC group) it is possible to establish higher immobilization levels compared to NTA-derivatized carboxymethyl dextran with the additional benefit of drastically reduced leaching, resulting in a practically drift-free baseline. Fragment screening by SPR and advanced application to GPCRs. A closer look at the His-protein association reveals that during the injection of the protein, there is a maximum in association followed by a slight dissociation during the injection. Discovery of fragments that target key interactions in the signal recognition particle (SRP) as potential leads for a new class of antibiotics. In previous approaches to establish FBDD assays using SPR, the ligand was covalently immobilized on the sensor surface with high immobilization levels to ensure that the protein bound stably to the sensor chip surface. If you are not sure which molecule will have the least non-specific binding, you can run a quick test to see. 0000008055 00000 n
startxref
Lata, S., Reichel, A., Brock, R., Tamp, R., & Piehler, J. 0000006785 00000 n
 Surface Plasmon Resonance (SPR) is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. 0000002277 00000 n
Surface Plasmon Resonance (SPR) is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. 0000002277 00000 n
 0000012772 00000 n
0000012772 00000 n
 Faoro, C., Wilkinson-White, L., Kwan, A. H., & Ataide, S. F. (2018). 4). The effective use of polydentate high-affinity Ni-NTA surfaces in SPR measurements of small molecules is vividly demonstrated in a publication by Byun et al.6. When working with, . Both immobilization methods lack the possibility to remove the bound ligand from the sensor surface which is critical, for example, when working with GPCRs or other sensitive proteins which often denature over long screening campaigns. Byun, J. Fragment-based drug discovery (FBDD) has become a preferred alternative to high-throughput screening (HTS) to improve the discovery of small-molecule drug candidates. 0000035529 00000 n
0000039104 00000 n
0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 350 mM EDTA, 0.005% Surfactant P20, pH 8.3 or 50 mM Imidazole. H^5)"1!avA${d,bsAv3#
Ic} 0 '
endstream
endobj
startxref
0
%%EOF
132 0 obj
<>stream
biotin and antigen epitopes) the affinity may vary with the microenvironment created by moieties adjacent to the His-tag. Here we present our investigation of three immobilization strategies (dual-His-tagged target protein, His-tagged streptavidin, and switchavidin) that combine the robustness of irreversible immobilization with the flexibility of reversible immobilization. 0000012625 00000 n
Faoro, C., Wilkinson-White, L., Kwan, A. H., & Ataide, S. F. (2018). 4). The effective use of polydentate high-affinity Ni-NTA surfaces in SPR measurements of small molecules is vividly demonstrated in a publication by Byun et al.6. When working with, . Both immobilization methods lack the possibility to remove the bound ligand from the sensor surface which is critical, for example, when working with GPCRs or other sensitive proteins which often denature over long screening campaigns. Byun, J. Fragment-based drug discovery (FBDD) has become a preferred alternative to high-throughput screening (HTS) to improve the discovery of small-molecule drug candidates. 0000035529 00000 n
0000039104 00000 n
0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% Surfactant P20, pH 7.4, 10 mM HEPES, 150 mM NaCl, 350 mM EDTA, 0.005% Surfactant P20, pH 8.3 or 50 mM Imidazole. H^5)"1!avA${d,bsAv3#
Ic} 0 '
endstream
endobj
startxref
0
%%EOF
132 0 obj
<>stream
biotin and antigen epitopes) the affinity may vary with the microenvironment created by moieties adjacent to the His-tag. Here we present our investigation of three immobilization strategies (dual-His-tagged target protein, His-tagged streptavidin, and switchavidin) that combine the robustness of irreversible immobilization with the flexibility of reversible immobilization. 0000012625 00000 n
 Overlay plot of two sensorgrams comparing immobilization capacity and stability of His, Figure 3. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.
Overlay plot of two sensorgrams comparing immobilization capacity and stability of His, Figure 3. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.  0
2022 XanTec bioanalytics GmbH | | , A) Rigid carboxymethyled dextran hydrogel with single NTA groups with immobilized His, B) Flexible HC hydrogel forming poly-NTA chelating cages for high affinity immobilization of His, Figure 1. Thus, when only a part of the ligand sites are occupied, the binding seems to be more stable than at high concentrations. Adding 250 M EDTA to the ligand sample may reduce non-specific binding. This is explained by rebinding effects (1),(3). Suitable ligand concentrations are typically below 200 nM. 0000014279 00000 n
In some cases, it is possible to dissociate the bound His-protein with Ni2+. 0000020535 00000 n
[Mr{S)tQe%\A}Cr4,/:"xP0iaA{:!C|[S`iJ!z*zze{ ldKe>8{[&*! 0000000016 00000 n
Such applications are highlighted in two examples that greatly increased throughput for the kinetic characterization of potent kinase inhibitors and kinetic profiling of covalent inhibitors. Compared to the standard CMD-NTA chemistry, these new coatings, which are available in 30, 200, 1000 and 1500 nm thickness, can improve the stability of captured His6-tagged ligands by 2-3 magnitudes, matching the high affinity of the recently-developed Tris-NTA4. The great news is that Nicoya carries a wide-variety of, It is optimal to use the molecule with the least amount of non-specific binding as the ligand. Accelerate your drug discovery with Alto. 83 0 obj
<>
endobj
114 0 obj
<>/Encrypt 84 0 R/Filter/FlateDecode/ID[<1BDAE8F6437043D4AFEAA7EB88C81E25><9E98CB40D29342FC83FF4000C8985B37>]/Index[83 50]/Info 82 0 R/Length 130/Prev 516836/Root 85 0 R/Size 133/Type/XRef/W[1 3 1]>>stream
119 0 obj<>stream
Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. Low affinity binding needs continuous rebinding to generate stable binding. The affinity (KD 10-6 M (1) of this interaction is commonly sufficiently high to allow detailed analysis of subsequent analyte binding.
0
2022 XanTec bioanalytics GmbH | | , A) Rigid carboxymethyled dextran hydrogel with single NTA groups with immobilized His, B) Flexible HC hydrogel forming poly-NTA chelating cages for high affinity immobilization of His, Figure 1. Thus, when only a part of the ligand sites are occupied, the binding seems to be more stable than at high concentrations. Adding 250 M EDTA to the ligand sample may reduce non-specific binding. This is explained by rebinding effects (1),(3). Suitable ligand concentrations are typically below 200 nM. 0000014279 00000 n
In some cases, it is possible to dissociate the bound His-protein with Ni2+. 0000020535 00000 n
[Mr{S)tQe%\A}Cr4,/:"xP0iaA{:!C|[S`iJ!z*zze{ ldKe>8{[&*! 0000000016 00000 n
Such applications are highlighted in two examples that greatly increased throughput for the kinetic characterization of potent kinase inhibitors and kinetic profiling of covalent inhibitors. Compared to the standard CMD-NTA chemistry, these new coatings, which are available in 30, 200, 1000 and 1500 nm thickness, can improve the stability of captured His6-tagged ligands by 2-3 magnitudes, matching the high affinity of the recently-developed Tris-NTA4. The great news is that Nicoya carries a wide-variety of, It is optimal to use the molecule with the least amount of non-specific binding as the ligand. Accelerate your drug discovery with Alto. 83 0 obj
<>
endobj
114 0 obj
<>/Encrypt 84 0 R/Filter/FlateDecode/ID[<1BDAE8F6437043D4AFEAA7EB88C81E25><9E98CB40D29342FC83FF4000C8985B37>]/Index[83 50]/Info 82 0 R/Length 130/Prev 516836/Root 85 0 R/Size 133/Type/XRef/W[1 3 1]>>stream
119 0 obj<>stream
Since the carboxyl and NTA sensor chip surfaces are negatively charged, a positively charged analyte will result in more non-specific binding. Low affinity binding needs continuous rebinding to generate stable binding. The affinity (KD 10-6 M (1) of this interaction is commonly sufficiently high to allow detailed analysis of subsequent analyte binding.  Figures 2 and 3 are showing the much higher stability of a captured His6-tagged ligand (a protein A/G fusion protein) on XanTecs poly-NTA surface NiHC1000M than on NTA-derivatized CMD hydrogel (mono-NTA). Such drift effects can easily exceed the specific signal when screening small molecules and thus represent a major problem. Fill out the form below to download a product brochure. This field is for validation purposes and should be left unchanged. Surface Plasmon Resonance biosensor analysis as a useful tool in FBDD. A., Van, K., Huang, J., Henning, P., Franz, E., Akimoto, M., & Melacini, G. (2020). Hale (5) describes the oriented immobilization of an antibody via the C-terminal part of the heavy chain (in IMAC not sensor chips). endstream
endobj
80 0 obj<. 0000006368 00000 n
If a small compound is used as the analyte and a large protein is used as the ligand, relatively large amounts of ligand must be on the sensor chip before a significant signal upon binding of the small compound can be seen.
Figures 2 and 3 are showing the much higher stability of a captured His6-tagged ligand (a protein A/G fusion protein) on XanTecs poly-NTA surface NiHC1000M than on NTA-derivatized CMD hydrogel (mono-NTA). Such drift effects can easily exceed the specific signal when screening small molecules and thus represent a major problem. Fill out the form below to download a product brochure. This field is for validation purposes and should be left unchanged. Surface Plasmon Resonance biosensor analysis as a useful tool in FBDD. A., Van, K., Huang, J., Henning, P., Franz, E., Akimoto, M., & Melacini, G. (2020). Hale (5) describes the oriented immobilization of an antibody via the C-terminal part of the heavy chain (in IMAC not sensor chips). endstream
endobj
80 0 obj<. 0000006368 00000 n
If a small compound is used as the analyte and a large protein is used as the ligand, relatively large amounts of ligand must be on the sensor chip before a significant signal upon binding of the small compound can be seen.  0000011552 00000 n
0000012184 00000 n
Drug Discovery Today: Technologies, 7(3), e181-e187. @@H
^g
5s/g@Y! wS*lNDQ#Ad23wA,
I[ <]>>
Sensor chip NTA (NTA: nitrilotriacetic acid) is designed to bind histidine-tagged molecules. Bayesian analysis of heterogeneity in the distribution of binding properties of immobilized surface sites. 0000020466 00000 n
To account for this well-known disadvantage, surface chemists at XanTec developed a poly-NTA sensor chip hydrogel coating based on a strongly hydrated and very flexible polycarboxylate polymer backbone. 0000003789 00000 n
However, a key requirement of SPR is the immobilization of the target protein to the surface of the sensor chip. (2014). For the best results, the histidine-tagged ligand should be purified prior to immobilization and prepared in eluent buffer. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.
0000011552 00000 n
0000012184 00000 n
Drug Discovery Today: Technologies, 7(3), e181-e187. @@H
^g
5s/g@Y! wS*lNDQ#Ad23wA,
I[ <]>>
Sensor chip NTA (NTA: nitrilotriacetic acid) is designed to bind histidine-tagged molecules. Bayesian analysis of heterogeneity in the distribution of binding properties of immobilized surface sites. 0000020466 00000 n
To account for this well-known disadvantage, surface chemists at XanTec developed a poly-NTA sensor chip hydrogel coating based on a strongly hydrated and very flexible polycarboxylate polymer backbone. 0000003789 00000 n
However, a key requirement of SPR is the immobilization of the target protein to the surface of the sensor chip. (2014). For the best results, the histidine-tagged ligand should be purified prior to immobilization and prepared in eluent buffer. SPR experiments typically consist of a ligand, the biomolecule that is coupled to the sensor surface, and the analyte, the biomolecule that is in solution and binds to the ligand.  We use cookies to help provide and enhance our service and tailor content and ads. To make this group of sensitive molecules accessible to FBDD, various attempts have been made to immobilize them reversibly via affinity based His6/nickel-nitrilotriacetic acid (NTA) coupling (Fig. 1). If you are having trouble with nonspecific binding, here are. Alternatively, biotinylated proteins were immobilized on streptavidin coated sensor surfaces with the inherent drawback that the analyte could non-specifically interact with the streptavidin. O'Shannessey (2) tested three buffers (Tris, Hepes, phosphate) and concluded that Hepes gives the best reproducible and sensitive results.
We use cookies to help provide and enhance our service and tailor content and ads. To make this group of sensitive molecules accessible to FBDD, various attempts have been made to immobilize them reversibly via affinity based His6/nickel-nitrilotriacetic acid (NTA) coupling (Fig. 1). If you are having trouble with nonspecific binding, here are. Alternatively, biotinylated proteins were immobilized on streptavidin coated sensor surfaces with the inherent drawback that the analyte could non-specifically interact with the streptavidin. O'Shannessey (2) tested three buffers (Tris, Hepes, phosphate) and concluded that Hepes gives the best reproducible and sensitive results.  0000005086 00000 n
0000005086 00000 n